Something in the water Part 1/2
7 juillet 2016 19:33 Laisser vos pensées
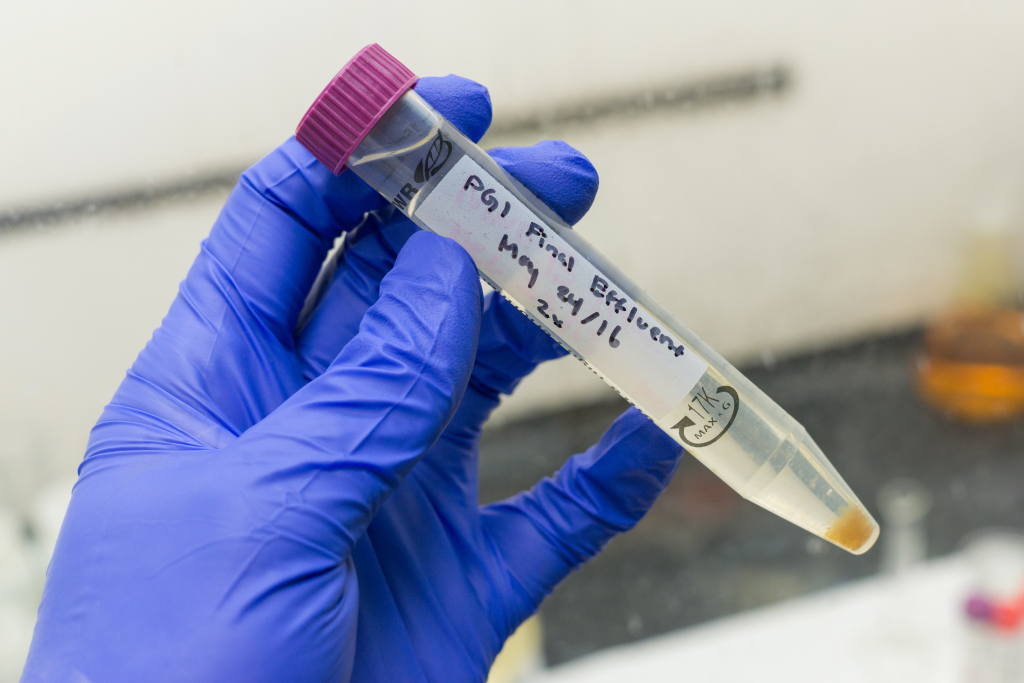
Back this week with a special unboxing for you guys. Just got this cooler from the Prince George Mill.
Let’s open it up and see what we have inside!
Bang bang!
Looks like we got a delivery of effluent samples from the mill for chemical analysis. Let’s find out what chemicals and biological compounds are present in each of these samples. We are mainly interested in the concentration of metals, anions – namely chloride and sulfate anions, and organic compounds in water. This week I will share with you how we perform a metal scan on our effluents.
Significance:
Why do we need to perform a metal scan on our effluents?
-
Environmental
The Kraft process requires large amounts of very caustic chemical during its production and poses a significant risk to the environment if it is not removed from the final effluent. Heavy metals such as lead, cadmium, and arsenic are very toxic to aquatic life and humans. The concentration of these heavy metals and other trace elements must be carefully monitored. The concentrations need to be below what is allowable by law.
-
Chemical Recovery
The benefit of the Kraft process is that it recovers most of the chemicals used in digestion. Sodium hydroxide and sodium sulfide are the main constituents of the white liquor that is used to extract the cellulose fibres from the woodchips. Depending on where in the process the sample is taken, the sodium and sulfide concentration can give an indication of how efficient the recovery process is.
Method:
Now, how do we measure the metals content in our samples? We use Inductively Coupled Plasma Optical Emission Spectroscopy (IC-OES) to determine the unknown concentrations of metals in our samples.
Our effluent samples are mixed with nitric acid and diluted with de-ionized water. Nitric acid is added to help dissolve any solids present and adds stability to the compounds in solution.
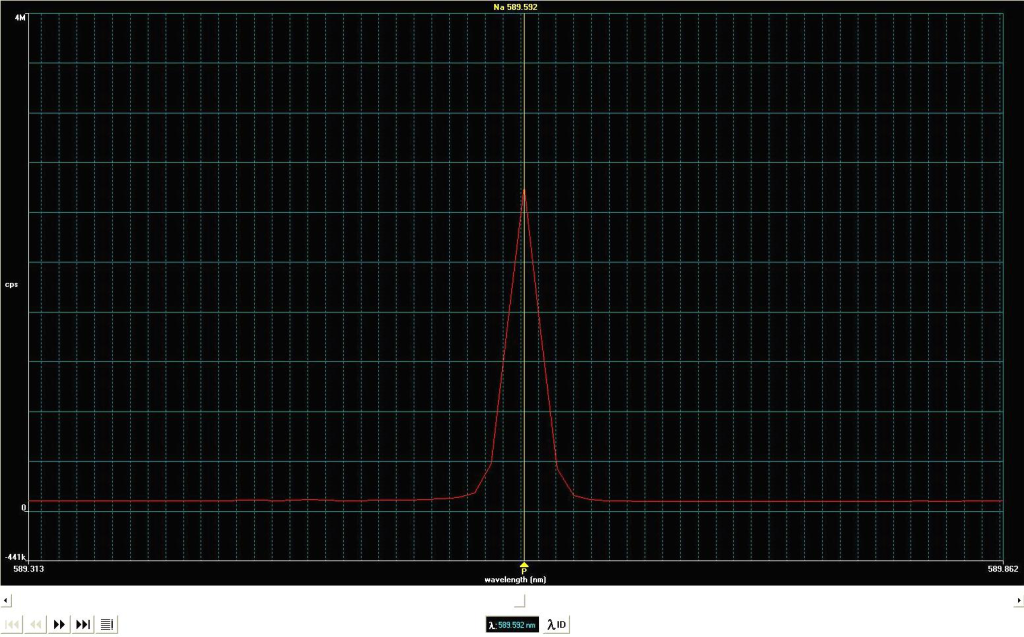
The sample is introduced as an aerosol into the 10,000°C argon plasma. The electrons are excited in higher energy levels and when they come back down, a light of specific wavelength is emitted and measured.
Procedure:
Firstly each of the samples that we receive are pipetted into 100 mL volumetric flasks and diluted with de-ionized water. 5 mL of concentrated nitric acid is added to make it a 5% nitric acid solution by volume.
Once all the samples are prepared, the diluted samples are transferred from the flasks into vials and loaded onto the auto sampler. At this point, the ICP has warmed up and finished its initialization. I enter the order that I want the instrument to measure the samples in a sequence file.
The auto-sampler probe first moves into the blank solution of de-ionized water and 5% nitric acid. The ICP measures the background concentration to subtract from subsequent samples. It then measures prepared standards with elements of known concentrations, as it will use this to generate the calibration curve to determine the unknown concentration in the samples. After calibration, the ICP proceeds to measure the samples.
Once the analysis is complete, a data file is generated with the concentration of all the elements of interest present in our samples. The concentration of an element is proportional to the area of the peak measured by the ICP.
Other ICP Applications:
The ICP is not only used for effluent testing. Other applications include quality control and customer enquiries such as contaminants. Pulp samples for examples are prepared for ICP analysis by ashing in a muffle furnace to remove any organics and dissolved with nitric acid. Perhaps explain what ashing is or save for next time
Low levels of transition metals such as manganese and iron are required for pulp quality, as these metals will catalyze the formation of harmful radicals in the oxygen and bleaching stages.
Electrical grade and tissue grade pulps require low total metals content.