Something in the Water Part 2/2
19 juillet 2016 16:46 Laisser vos pensées
This week we will be concluding our effluent testing. Two other tests that we do in addition to the metals scan include measuring the chemical oxygen demand (COD), and the amount of chloride and sulfate anions.
Chemical Oxygen Demand:
Significance:
The chemical oxygen demand (COD) test is commonly used to indirectly measure the amount of organic compounds in water. Like humans, most aquatic life require oxygen to survive. Normally oxygen gets dissolved in water through diffusion from the air, the aeration of water in rapids and waterfalls, and photosynthesis of phytoplankton. When a large amount of organic waste gets released into the water, it decreases the available dissolved oxygen for other organisms. Increased organics can also cause algae blooms, which further reduce the amount of dissolved oxygen.
Method:
We analyze the COD content of our samples using colorimetric analysis. In other words, the concentration of organic compounds are measured based on the colour of the solution in the presence of a colouring reagent. The greener the sample is, the more organics that are present.
Procedure:
The first step in the analysis is to dilute any samples that are known to have large amounts of organics. The sample vials can only be used to analyze samples within 0-1500 mg/L COD. The reagents inside the vials contain strong oxidizing agents.
Next, we pipette 2 mL of our prepared solutions into the reagent vials. A sample of potassium acid phthalate (KHP) standard is prepared to use as an accuracy check.
We then place all the vials into the reactor where it is heated to 150°C for two hours. The vials are then transferred to a rack and cooled to room temperature.
Now that the samples are prepared, they are ready to by analyzed using the colorimeter. After zeroing the device, the KHP vial is analyzed for an accuracy check. Each vial is cleaned with a Kimwipe (a fibre free tissue) and inserted into the cavity of the device and a light shield is placed on top. If the instrument is properly calibrated, the COD content should be 500 mg/L. After calibration, each sample is analyzed in the same fashion.
High Performance Liquid Chromatography (HPLC)
Significance:
Chloride (Cl–) and sulfate (SO32-) anions are two important compounds to the Kraft Process. They are added during digestion in the bleaching stages of the process. Measuring the amounts of these compounds in various stages of the process gives an indication of how efficient the recovery process is. Excess sulfate in water can cause scale buildup in pipes and have a laxative effect on humans if consumed.
Method:
High-performance liquid chromatography is an analytical technique used to separate, identify and quantify components in a mixture. A pressurized liquid mobile phase carries the sample through a solid phase column and is analyzed at a detector at end of the column. Each component in the mixture interacts differently with the column, which leads to the separation as they leave the column. The area underneath each peak in the chromatograph tells us the concentration of the compound in the mixture.
Procedure:
Sample preparation begins with diluting our samples with Nanopure water. Nanopure water is de-ionized water that is further filtered to remove any remaining anions. After dilution, 1.5 mL of each sample is transferred to samples and placed into the auto sampler tray. Standards of the known concentrations of chloride and sulfate are also added to the tray.
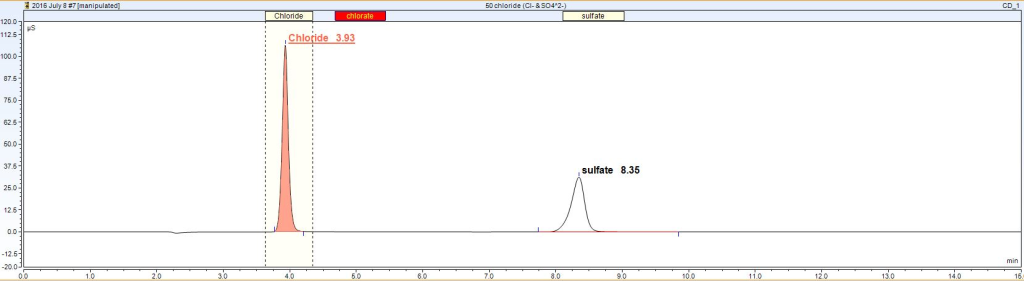
Once the trays have been loaded with our sample vials, the tray is placed into the autosampler where the sampling needle is inserted into each of the vials. The sample is pumped into the HPLC where the mobile liquid carries the sample through the column. As seen on the graph below, chloride is first to elute through the column followed by sulfate.
The area underneath each peak is precisely the concentration of each anion present in the sample in mg/L (or parts per million). If the sample was diluted, the measured concentration needs to be multiplied by the dilution factor to determine the actual concentration present in each of the samples.
That concludes our weekly effluent testing!
– TW